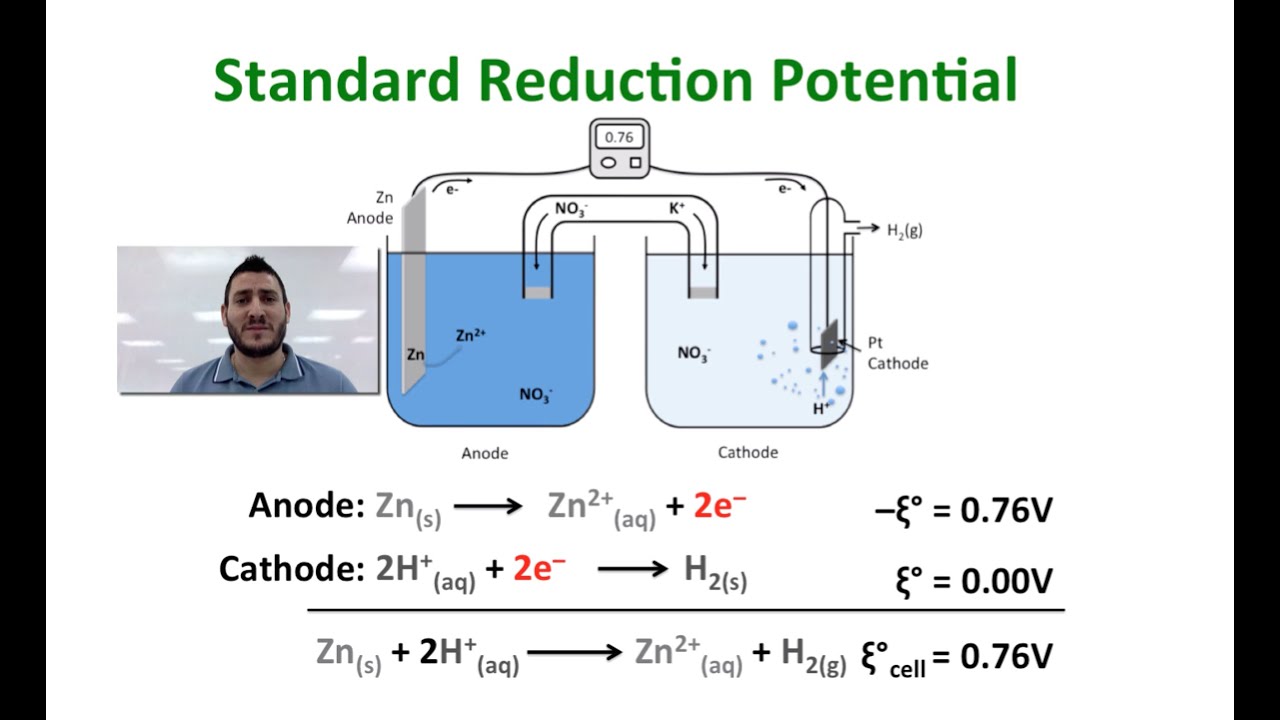
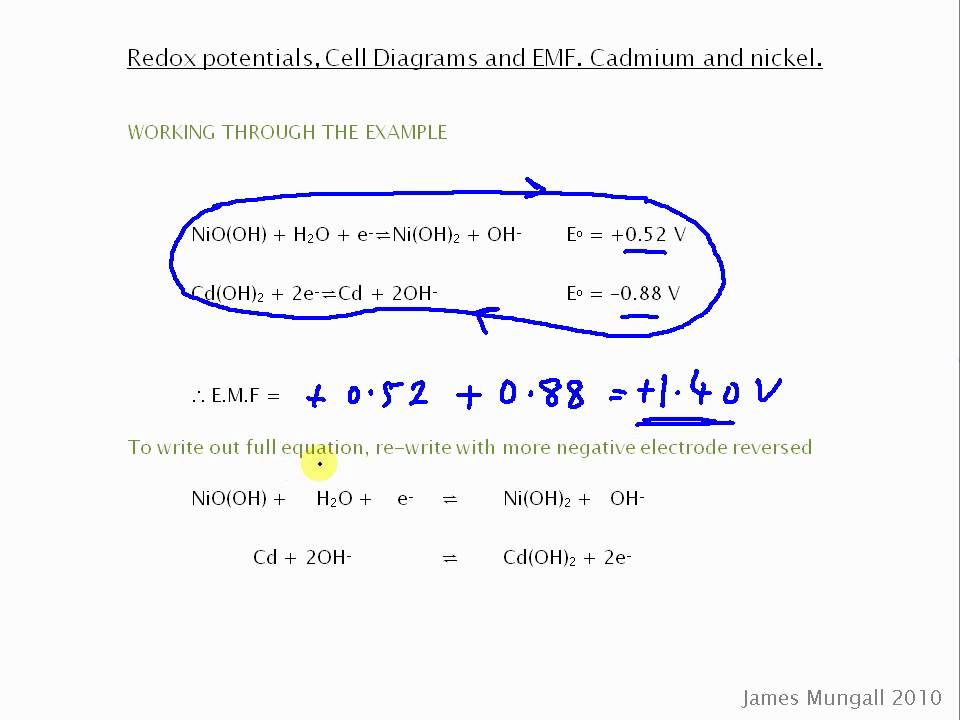
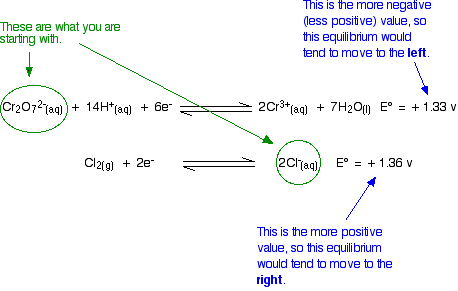
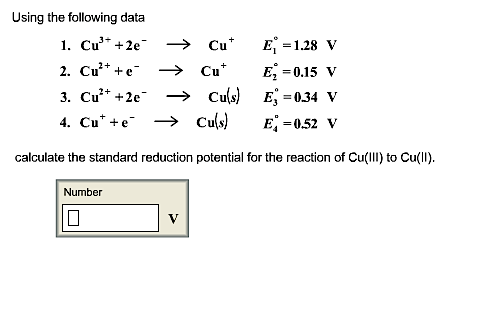
The standard reduction potential for the half cell: NO3^-(aq.) + 2H^+(aq.) + e^ - → NO2(g) + H2O is 0.78 V. Calculate the reduction potential in 8M H^+ .

Standard Reduction Potentials for Oxygen and Carbon Dioxide Couples in Acetonitrile and N,N-Dimethylformamide | Inorganic Chemistry
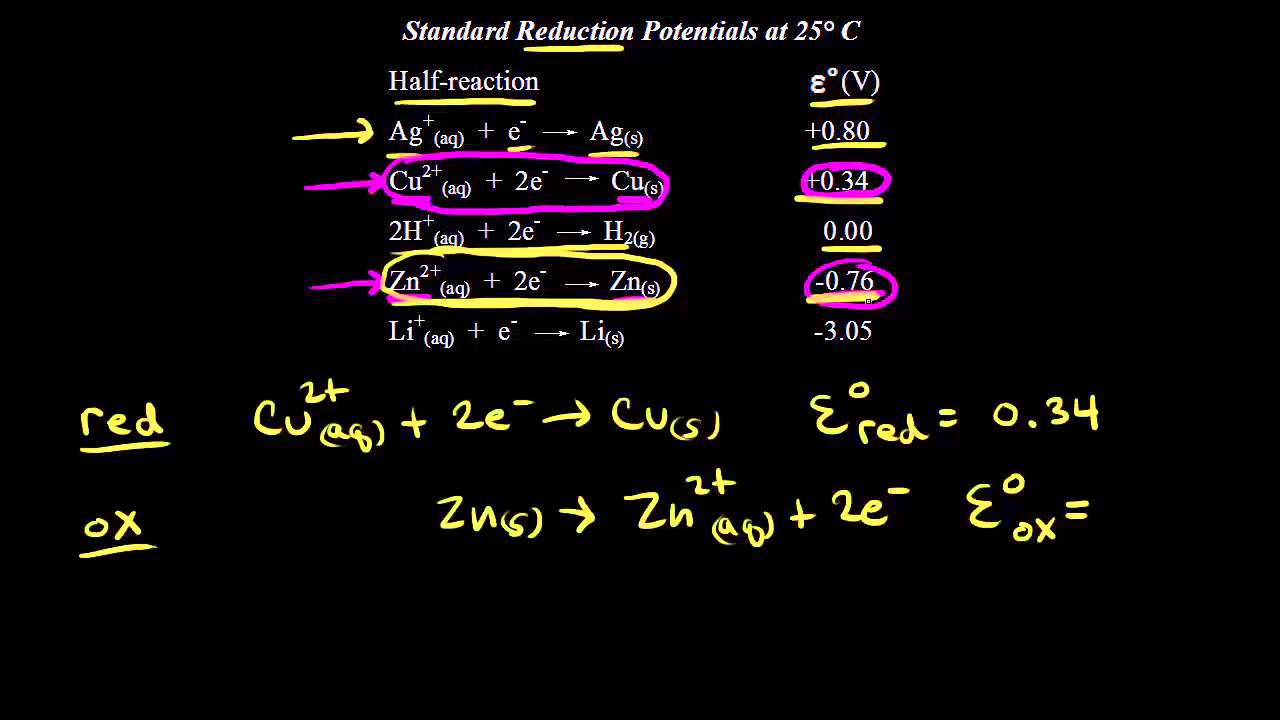
Standard reduction potentials | Redox reactions and electrochemistry | Chemistry | Khan Academy - YouTube
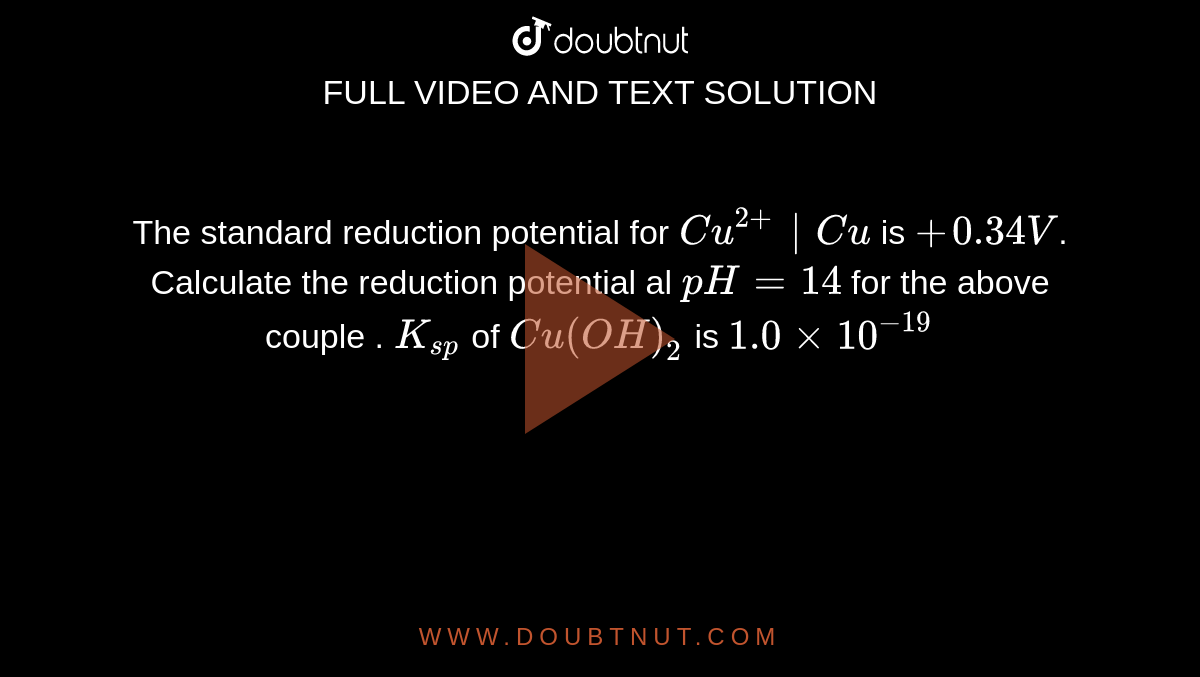
The standard reduction potential for Cu^(2+)|Cu is +0.34V. Calculate the reduction potential al pH=14 for the above couple . K(sp) of Cu(OH)(2) is 1.0xx10^(-19)

The standard reduction potential for `Cu^(2+)|Cu` is `+0.34V`. Calculate the reduction potential... - YouTube

Calculation of Standard Reduction Potentials of Amino Acid Radicals and the Effects of Water and Incorporation into Peptides. | Semantic Scholar
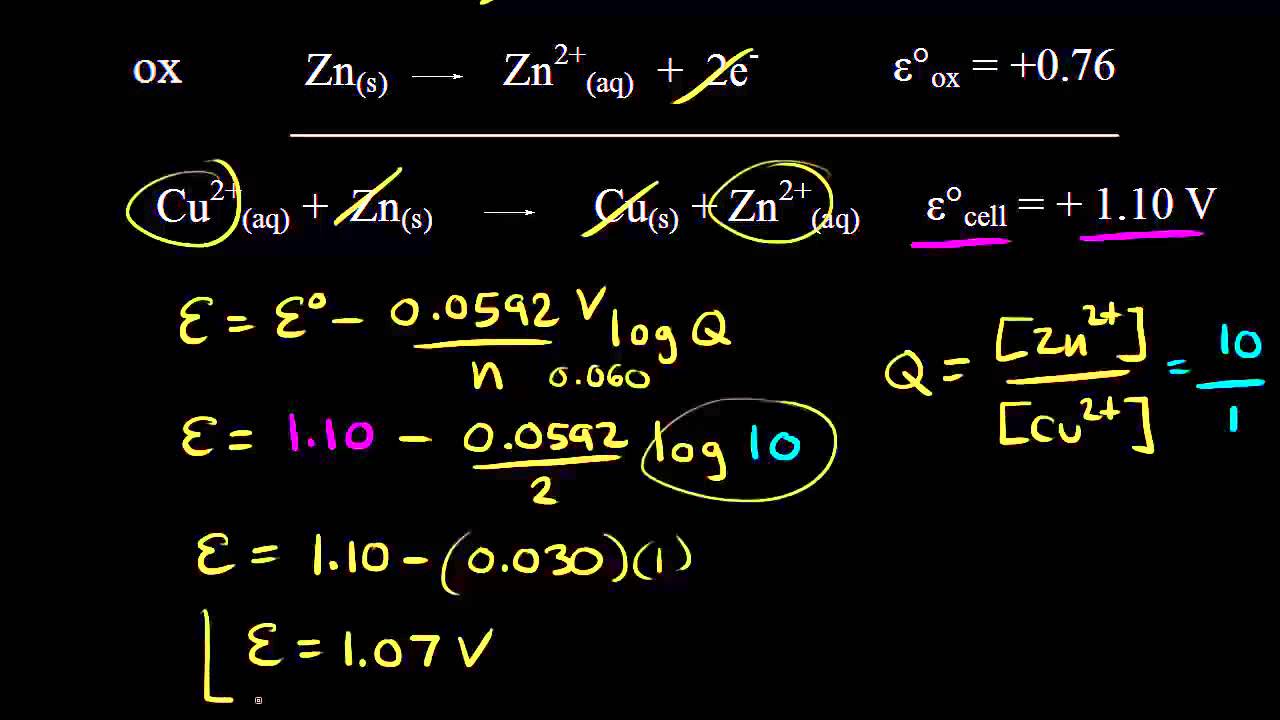
Using the Nernst equation | Redox reactions and electrochemistry | Chemistry | Khan Academy - YouTube
2 The standard half reduction potential of Ag+|Ag is 0.79V is 25^° C. Given the experimental value Ksp=1.5 10* 10 for AgCl, calculate the standard half cell reduction potential for the Ag|AgCl

Question Video: Calculating a Cell Potential from Standard Electrode Potentials of Cadmium and Nickel | Nagwa