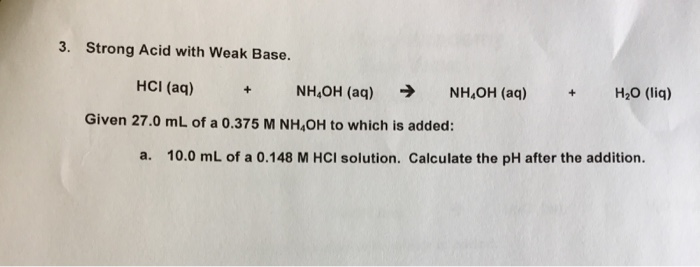
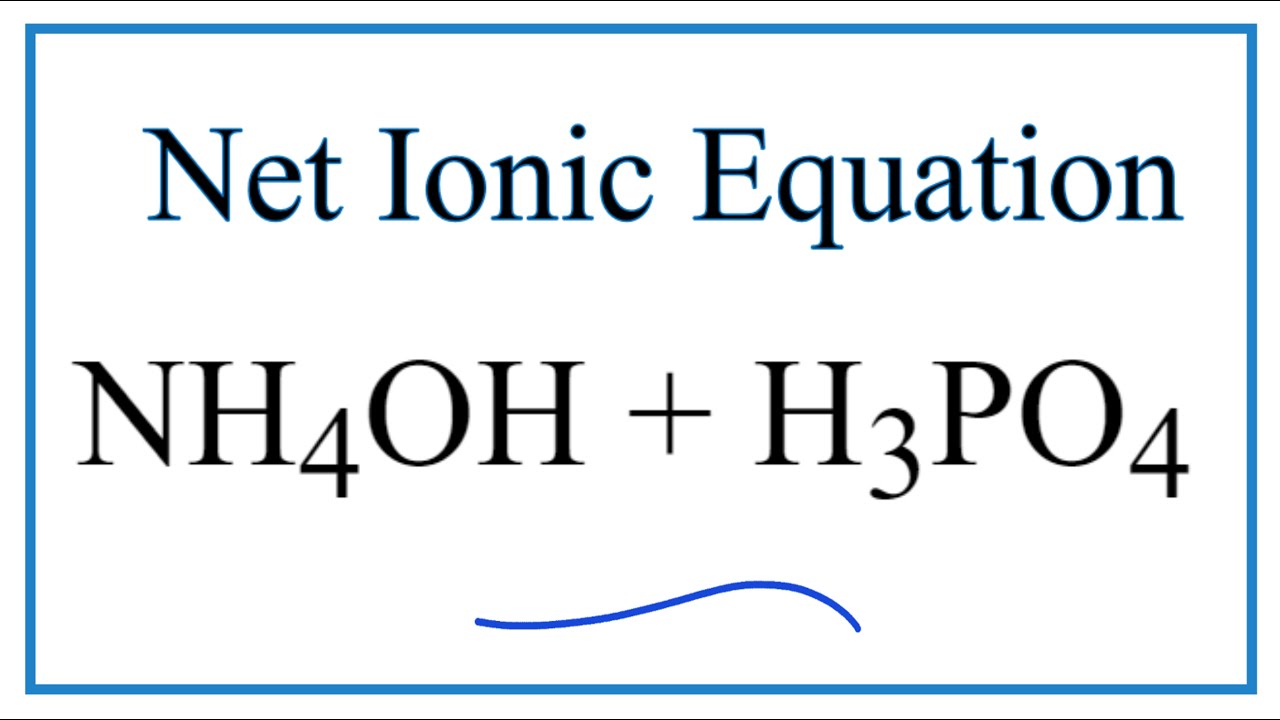Question Video: The Net Ionic Equation for the Neutralization Reaction between Ammonium Hydroxide and Hydrochloric Acid | Nagwa
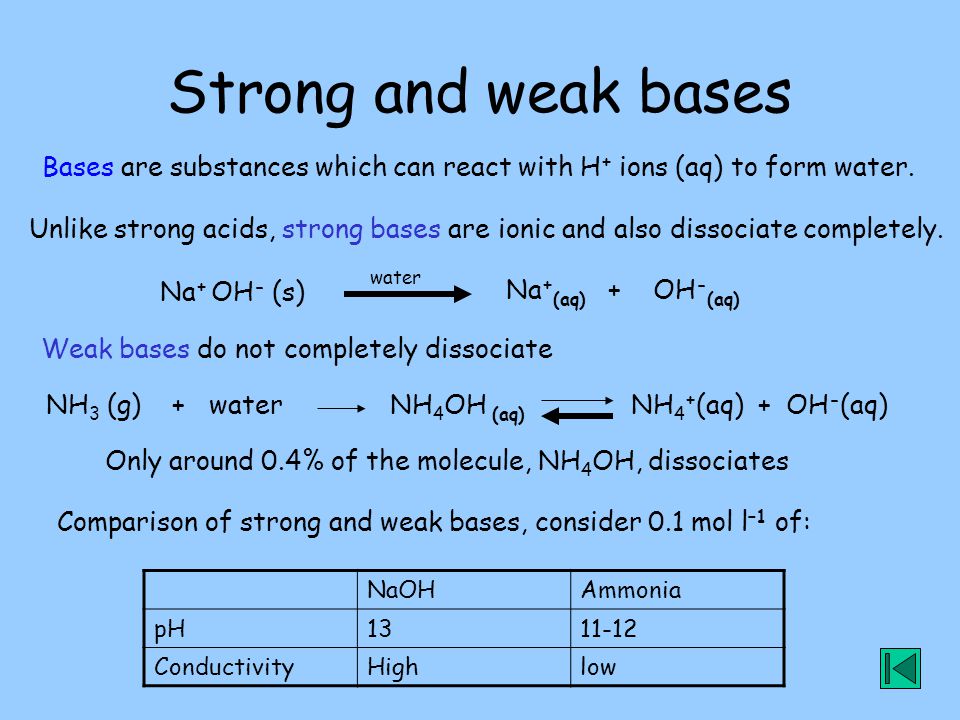
Chemical Equilibrium Equilibrium, strong and weak acids and bases, solutions, concentration and pH. - ppt video online download

The enthalpy of neutralisation of NH4OH with HCl is - 51.46kJ/ mol^-1 and the enthalpy of neutralisation of NaOH with HCl is - 55.90kJ/ mol^-1 .The enthalpy of ionisation of NH4OH is: