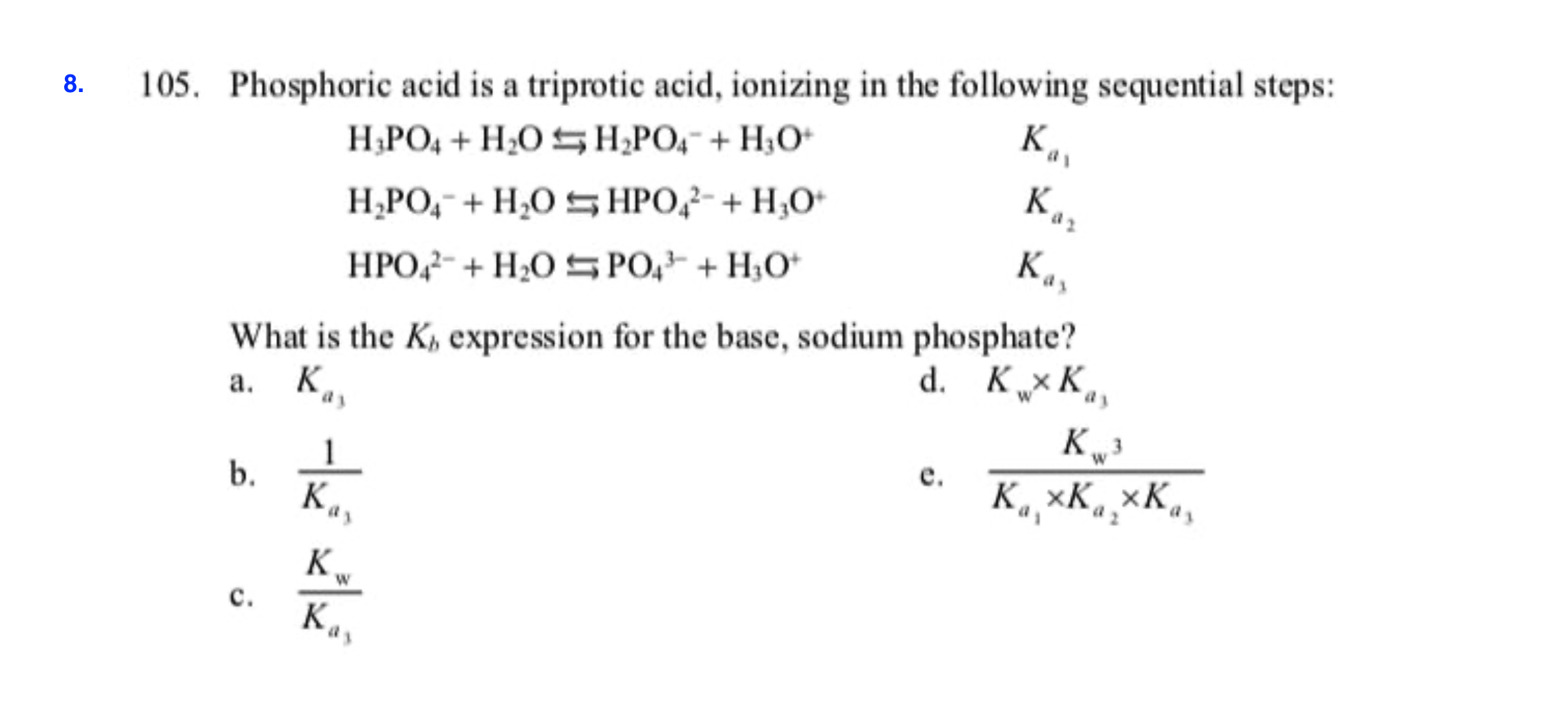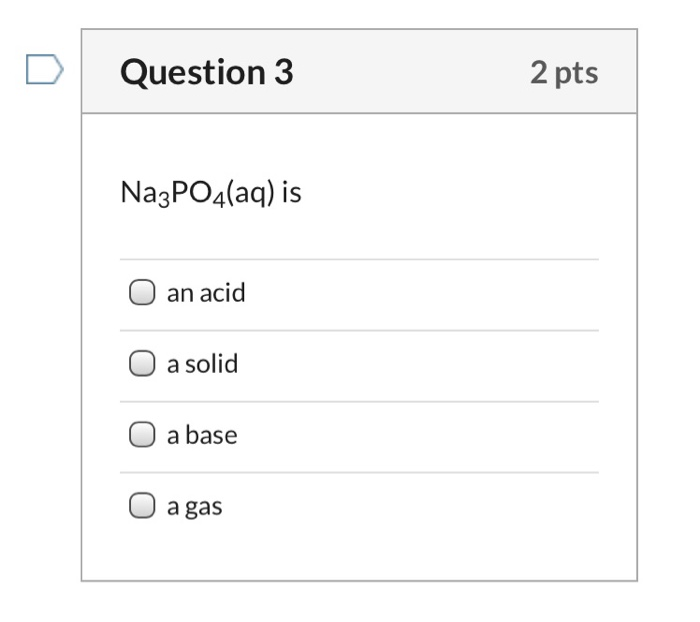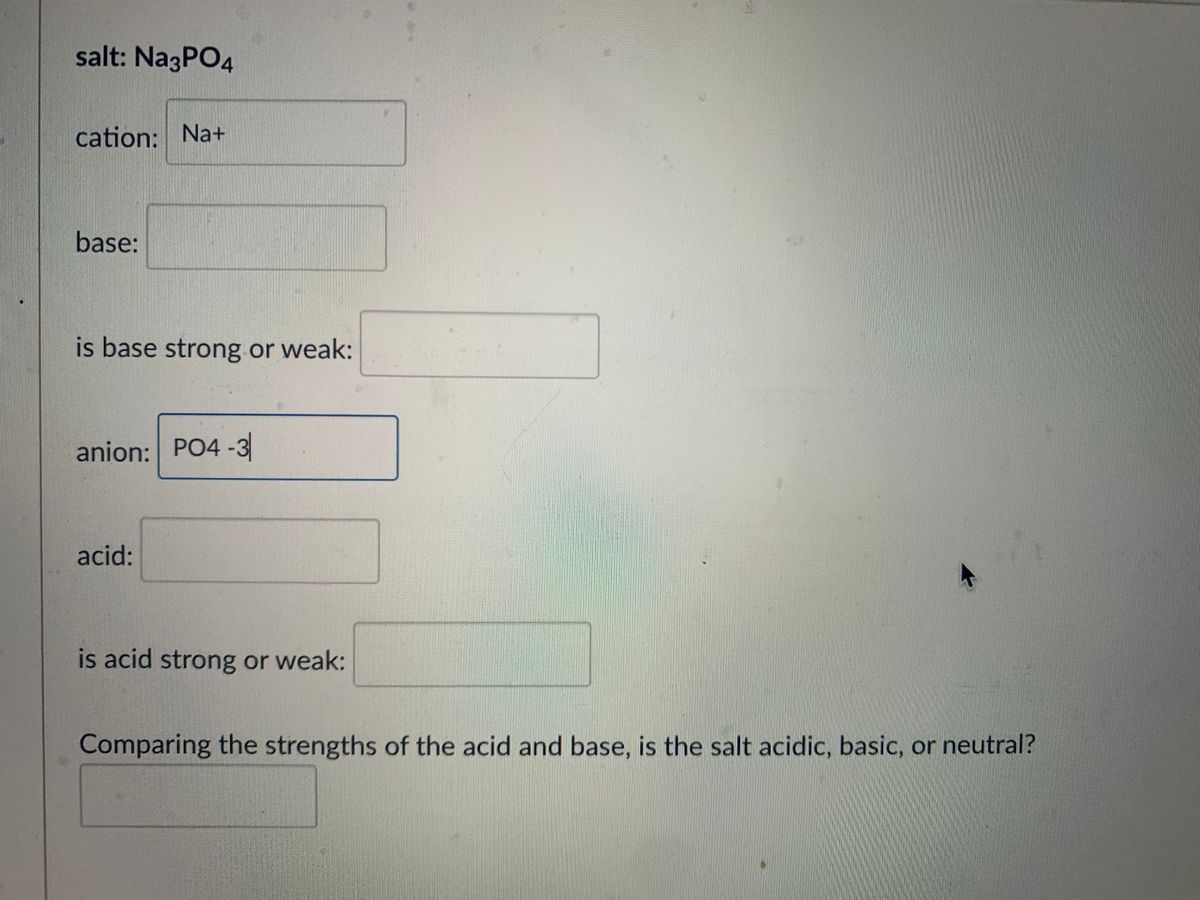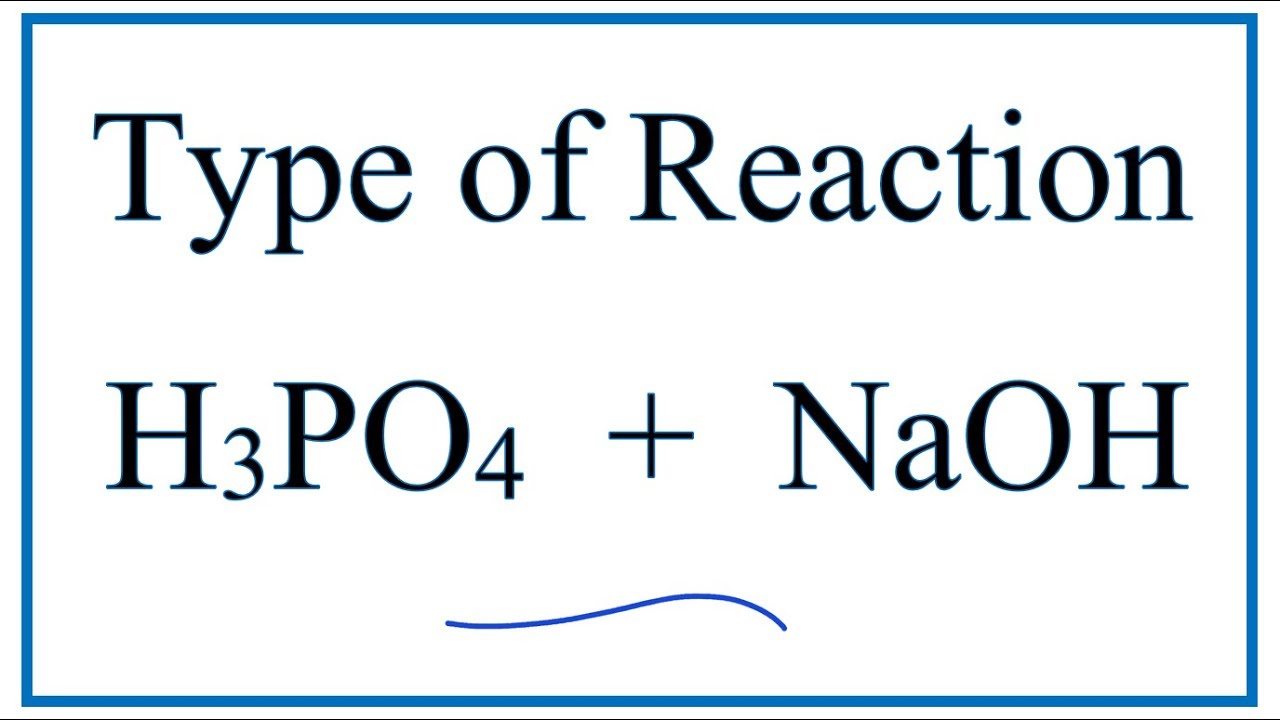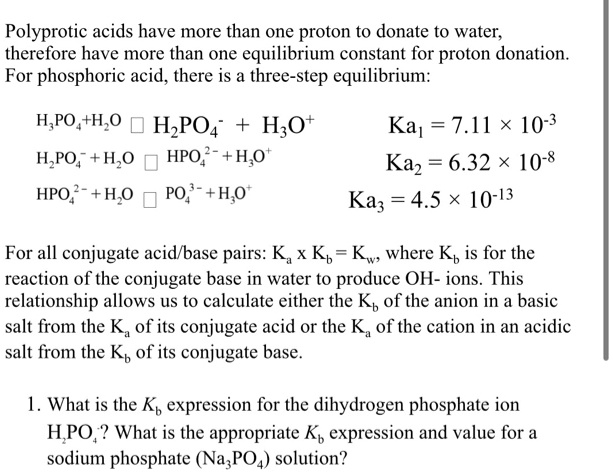
SOLVED: Polyprotic acids have more than one proton to donate to water; therefore have more than one equilibrium constant for proton donation For phosphoric acid, there is a three-step equilibrium: H,PO +H,o
![Expert Answer] classify the following salts as neutral , acidic or basic. also,write their name. a) - Brainly.in Expert Answer] classify the following salts as neutral , acidic or basic. also,write their name. a) - Brainly.in](https://hi-static.z-dn.net/files/d3d/3e43c78b8d42fd1bf93f75db19f306ce.jpg)
Expert Answer] classify the following salts as neutral , acidic or basic. also,write their name. a) - Brainly.in