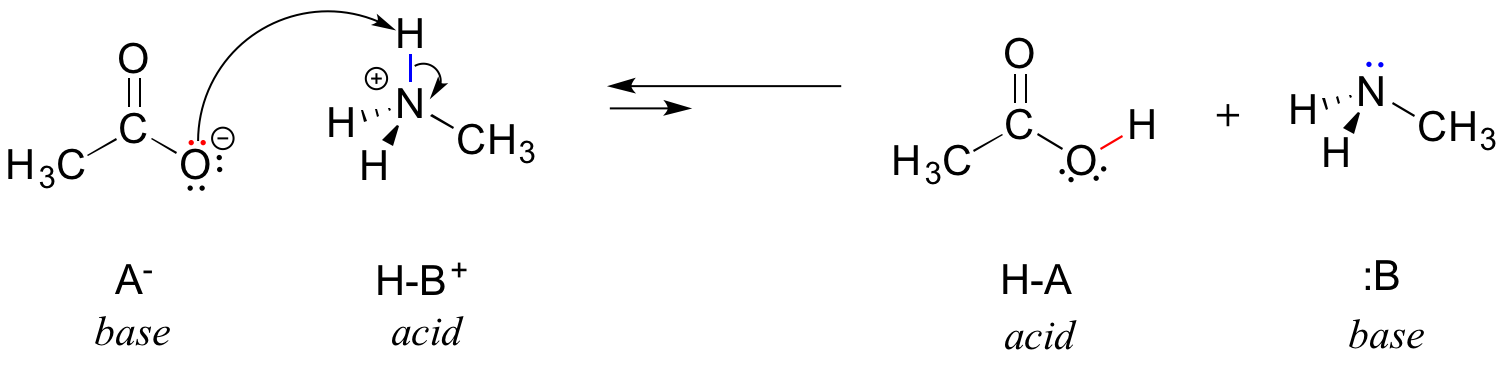
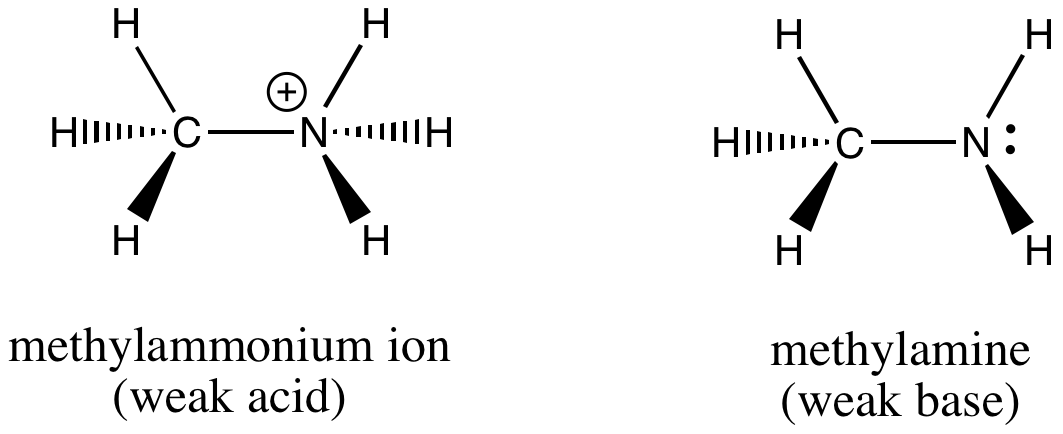
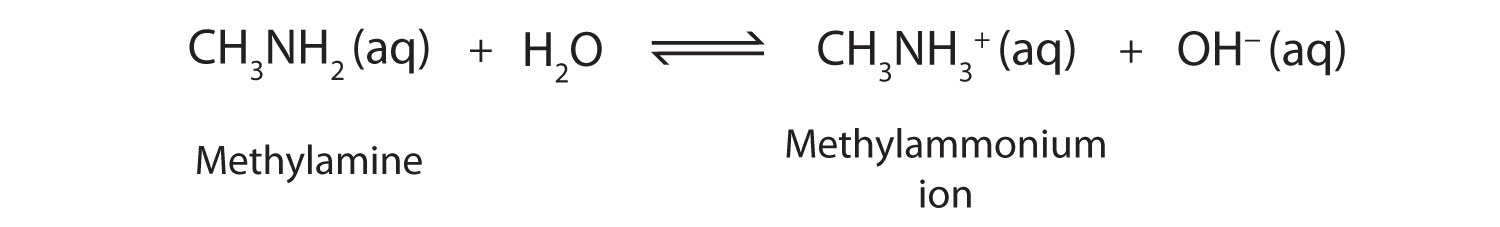
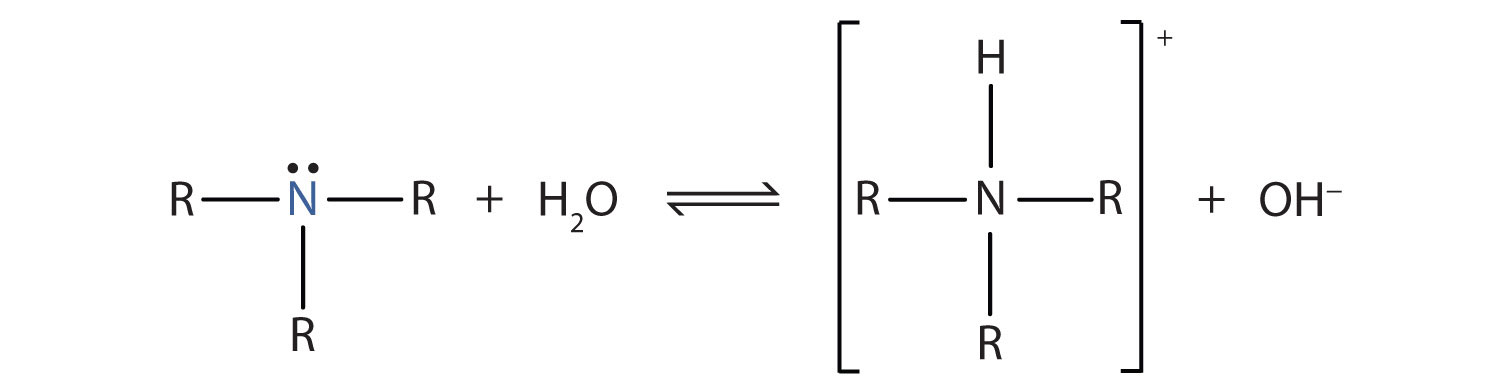
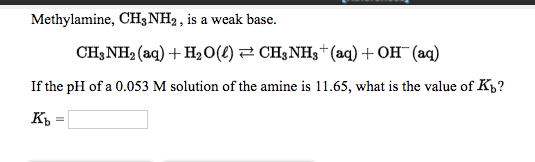Draw the Lewis structure for the conjugate acid and base from the reaction of methylamine with a generic base (B:-). Include all lone pairs of electrons and any nonzero formal charges.
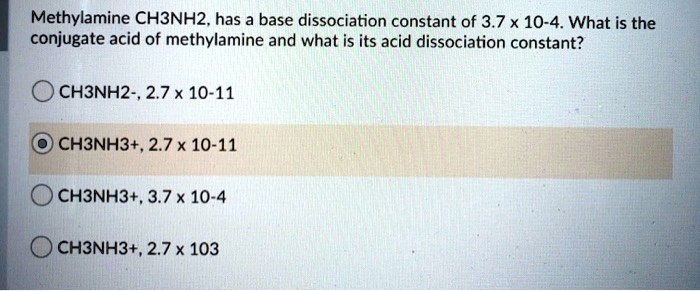
SOLVED: Methylamine CH3NH2, has a base dissociation constant of 3.7 x 10-4. What is the conjugate acid of methylamine and what is its acid dissociation constant? CH3NHZ -, 2.7 x 10-11 CH3NH3+,2.7