Dipea Nndiisopropylethylamine Hunigs Base Molecule Skeletal Stock Vector (Royalty Free) 1093026992 | Shutterstock
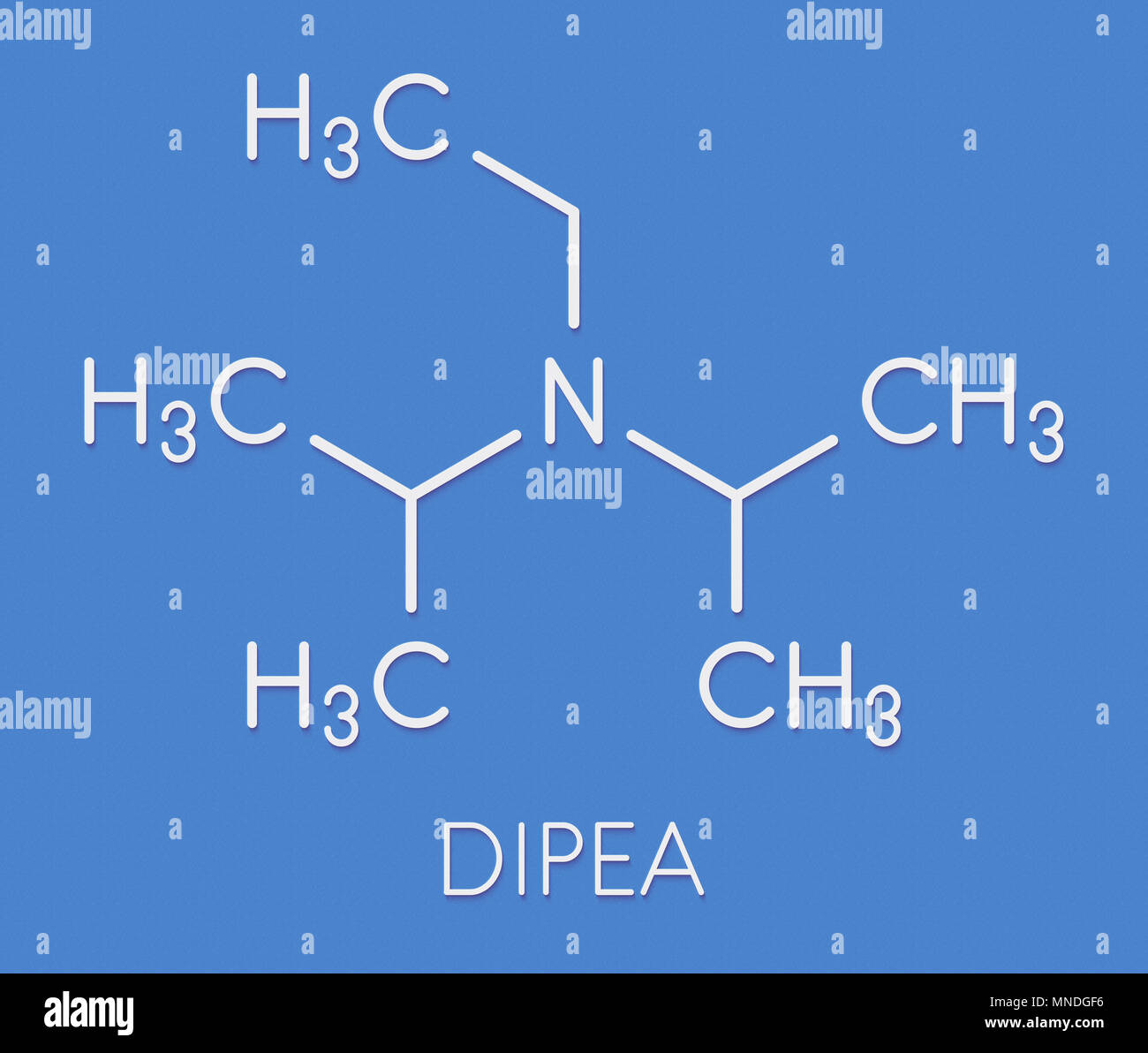
DIPEA (N,N-diisopropylethylamine, Hunig's base) molecule. Stylized skeletal formula (chemical structure): Atoms are shown as color-coded circles: hydrogen (hidden), carbon (grey Stock Photo - Alamy

DIPEA-Promoted Reaction of 2-Nitrochalcones with Elemental Sulfur: An Unusual Approach to 2-Benzoylbenzothiophenes | Organic Letters

Electrochemical Formation of Cinnamaldehyde by the Electrolyte System N,N‐ Diisopropylethylamine and 1,1,1,3,3,3‐Hexafluoropropan‐2‐ol - Imada - 2020 - ChemElectroChem - Wiley Online Library

DIPEA (N,N-diisopropylethylamine, Hunig's base) molecule. Stylized skeletal formula (chemical structure): Atoms are shown as color-coded circles: hydrogen (hidden), carbon (grey Stock Photo - Alamy

Scheme 4 Reagents and conditions: a) aryl alkyne, CuI, DIPEA, THF; b)... | Download Scientific Diagram

Scheme 40. Application of N,N-Diisopropylethylamine (DIPEA) for the... | Download Scientific Diagram
N,N-Diisopropyléthylamine (DIPEA), 2.5 l, cas.number.title.metatag 7087-68-5 | Réactifs de couplage | Synthèse peptidique | Produits chimiques organiques & bioorganiques | Produits chimiques | Carl Roth - France