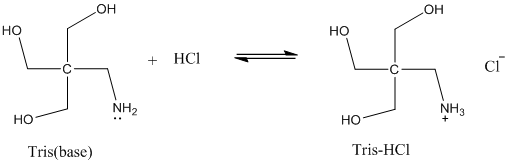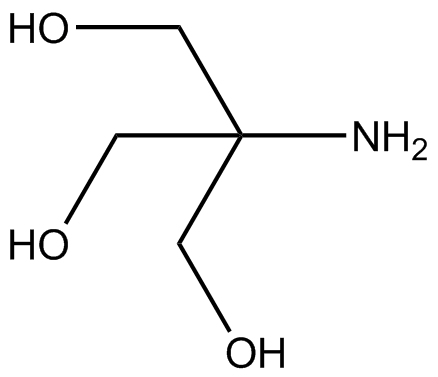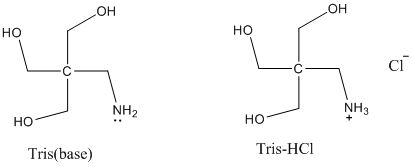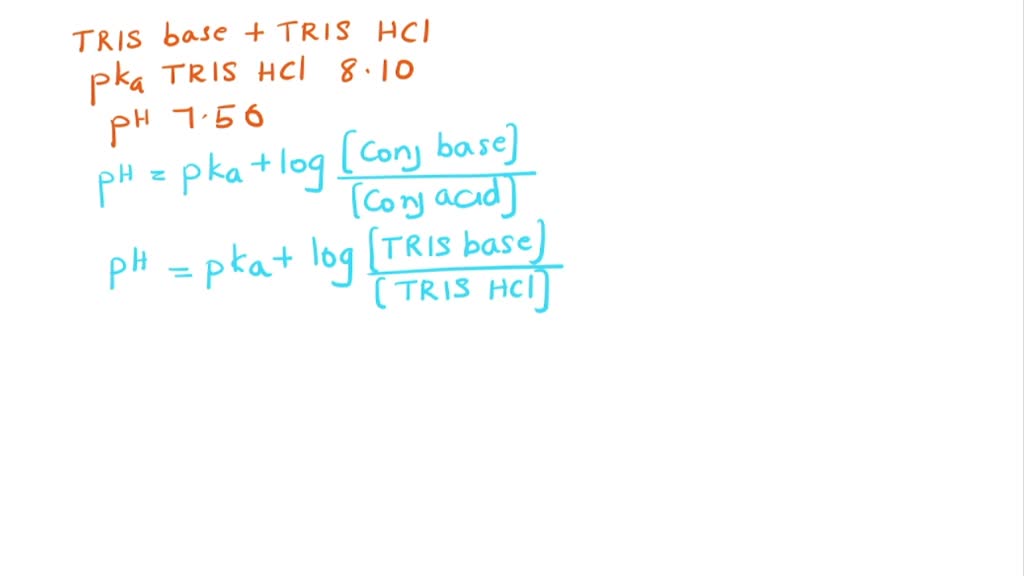
SOLVED: Question 35 3 pts A buffer is made from a mixture of TRIS base and TRIS-HCI The pKa of TRIS-HCl is 8.10. What is the ratio of TRIS base to TRIS -HCI
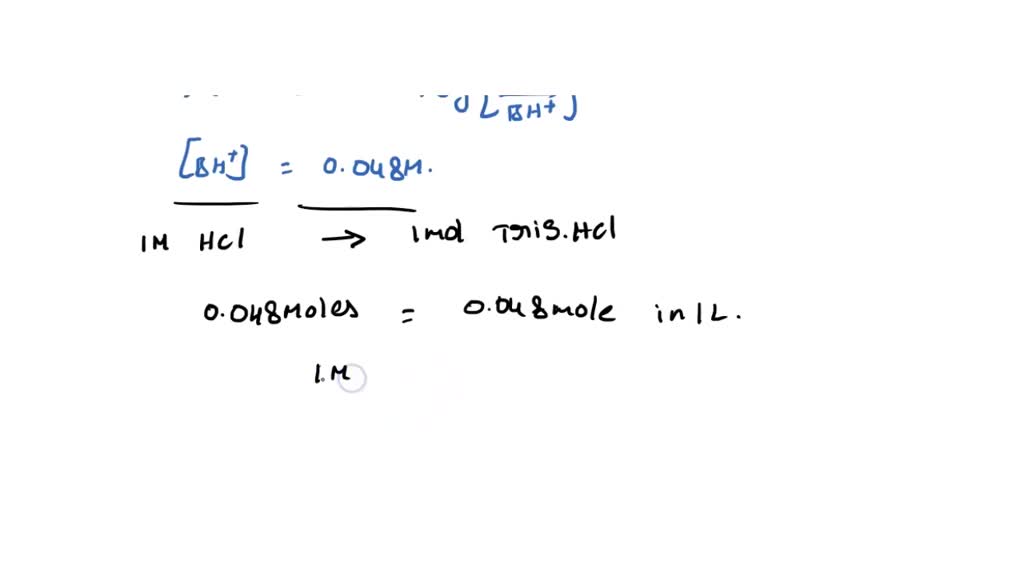
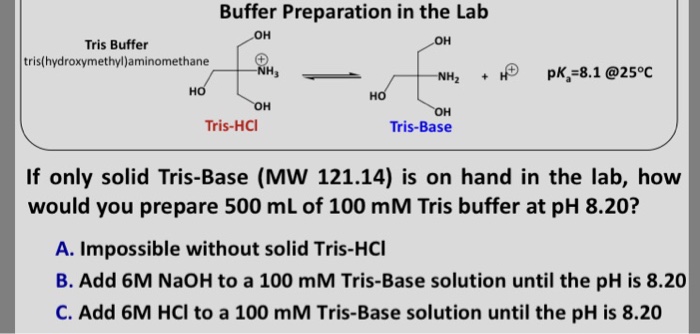
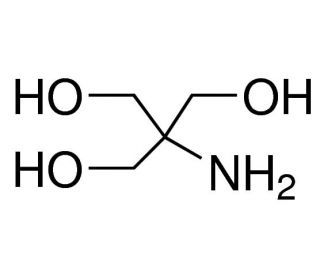
SOLVED: Calculate how you world prepare 100 mL of a 0.1 M TRIS-HCl buffer, pH 7.4 using solid TRIS base (tris-(hydroxymethyl)-aminomethane) (MW 121.1, pKa 8.08). You may find TRIS under several different

New insights into the effect of Tris-HCl and Tris on corrosion of magnesium alloy in presence of bicarbonate, sulfate, hydrogen phosphate and dihydrogen phosphate ions - ScienceDirect

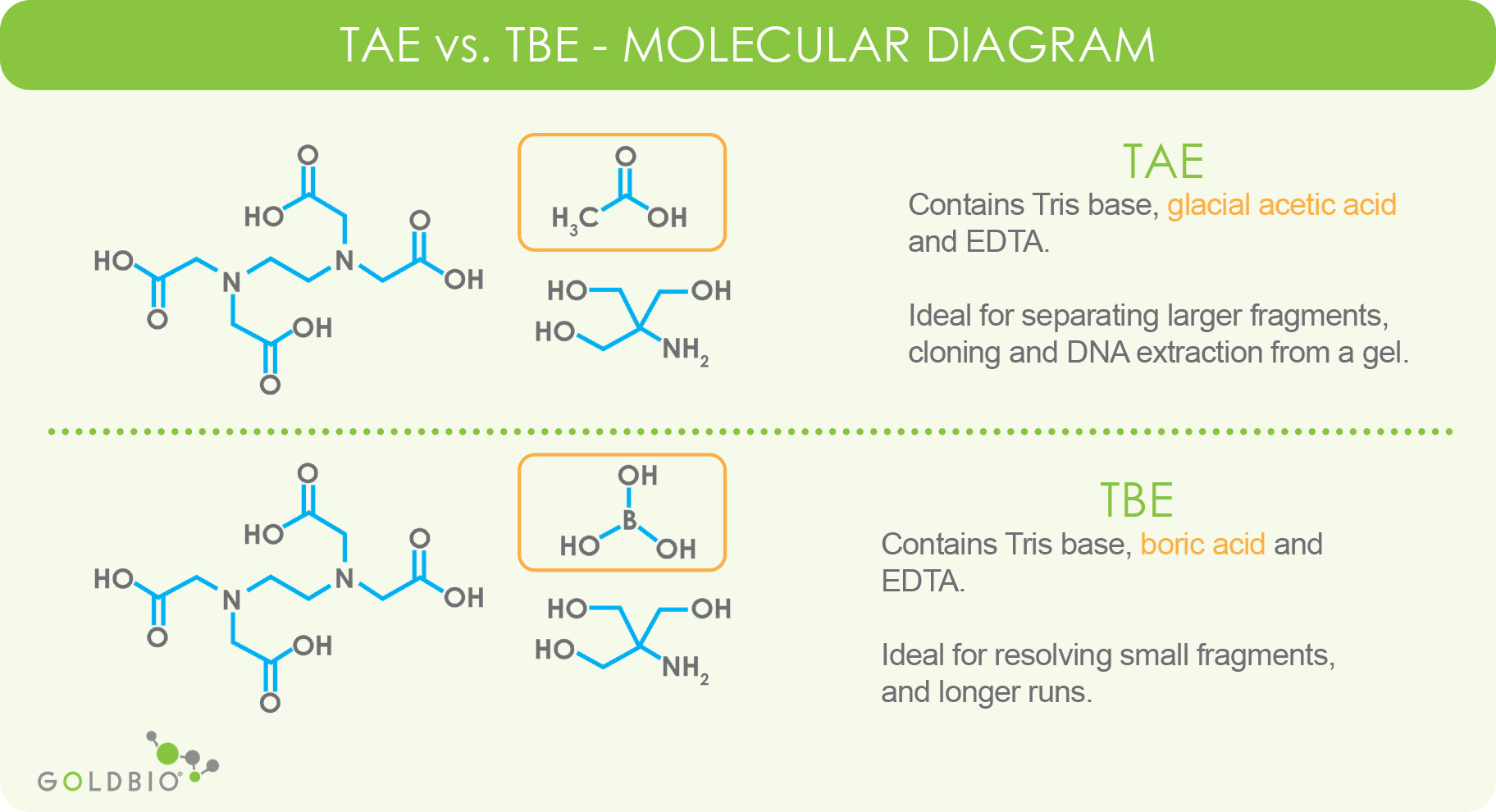
Scheme 2. Structures of Tris, TrisHCl and etilendiaminotetraacetic acid (H4EDTA) : pH and Acid-Base Equilibrium Calculations via a Matrix Representation of Solutions of Acids and/or Bases : Science and Education Publishing
![BT156] 2M Tris-HCl, pH 9.5 | Biosolution BT156] 2M Tris-HCl, pH 9.5 | Biosolution](http://biosolution.cafe24.com/wp-content/uploads/2015/07/BT066-Tris-HCl.jpg)